Alkenes are unsaturated hydrocarbons, which, like alkanes, form a homologous series. However, unlike alkanes, alkenes have one multiple bond – a double bond – between carbon atoms. The remaining ones are single bonds, but the occurrence of even one multiple bond in a molecule makes the hydrocarbon an unsaturated compound.

The structure of alkenes
The C-C bond between atoms in saturated hydrocarbons causes each of them to have a sp3 hybridisation. In alkenes, which have C=C bonds, there occurs a sp2 hybridisation. This property causes the structure of compounds with double bonds to be a trigonal form. Its 3 sigma bonds are in the same plane and face the corners of the equilateral triangle, the centre of which is the carbon atom. In contrast, pi bonds result from the overlapping of a non-hybrid p orbital in a direction perpendicular to the triangle, which is formed from hybrid orbitals and the orbitals of similar symmetry of the neighbouring atom. The simplest construction of the group, and the first compound in the alkene homologous series, is ethene. Methane, a compound that is the first of the alkanes, could not form a multiple bond.
An example of the structure of the alkene
Let’s take the simplest compound of the group, ethene, and examine its structure. We know that the carbon atoms in the molecule of ethene have a sp2 hybridisation, so the molecule is flat. The angles between bonds in this layout are 120o. The four bonds between the carbon and hydrogen atoms present in the structure C-H are sigma δSP-s bonds with a head-on overlap. There are also homonuclear bonds formed between the carbon atoms. One is C-C δsp-sp and the other is C-C πp-p, which is formed from non-hybrid p orbitals.
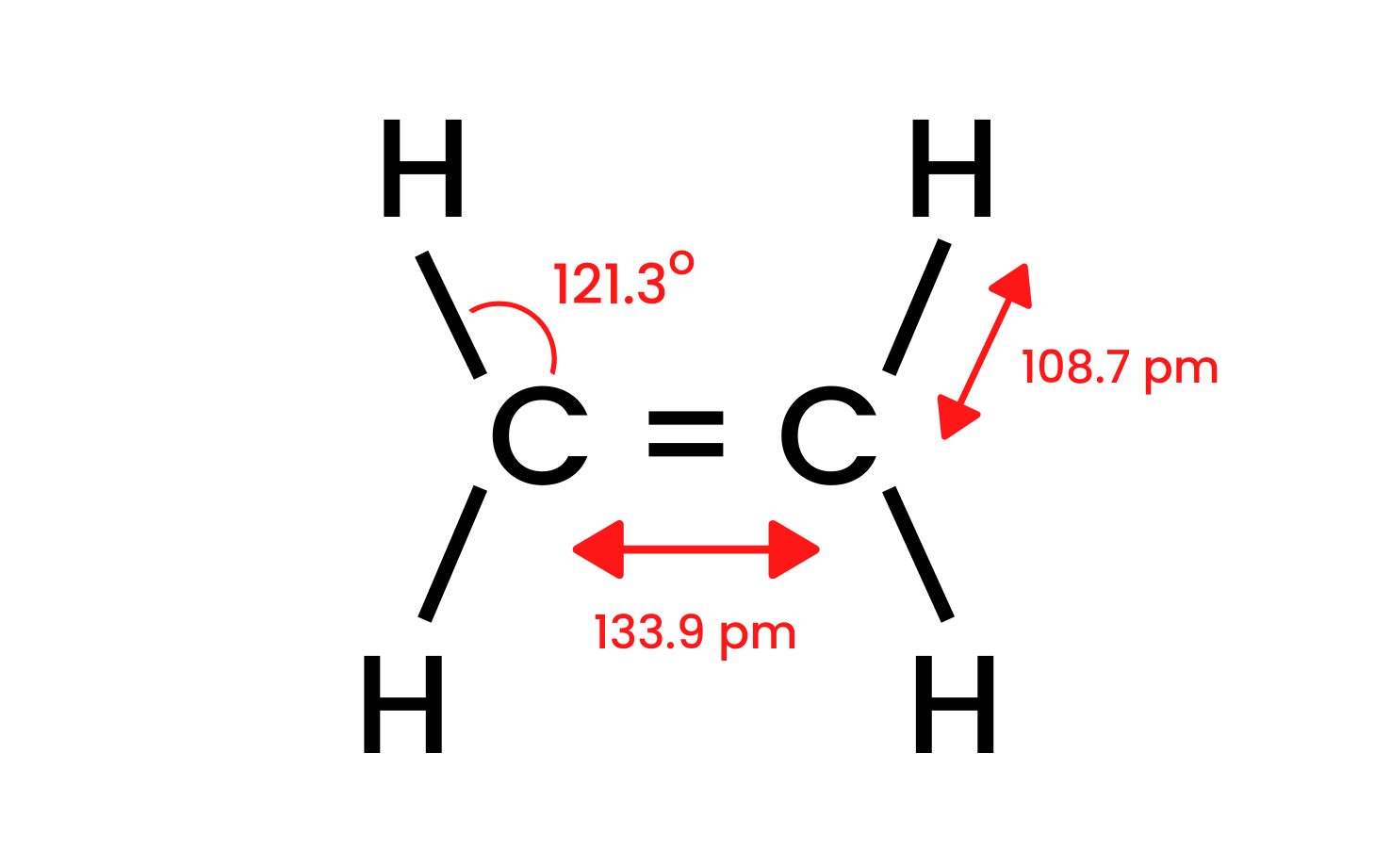
Figure 1. The structure of ethene
Alkene homologous series
A homologous series is a group of chemical compounds that have a very similar chemical structure and properties. Such substances can also be written with a common molecular formula.
The general formula for alkenes can be written as CnH2n. Structurally, it looks like this:
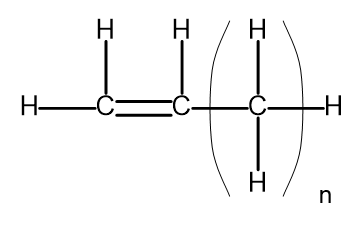
Figure 2. General structural formula for alkenes
Like saturated hydrocarbons, alkenes also form their own homologous series, which begins with ethene and ends with decene. The name of the alkene is derived from the name of an analogous alkane with the same number of carbon atoms in the molecule. The difference is in the suffix, which is –ane for alkanes, whereas for alkenes it is –ene, for example pentane/pentene.
| Number of carbon atoms in the chain | Alkane formula | Alkane name | Alkene formula | Alkene name |
| 2 | C2H6 | Ethane | C2H4 | Ethene |
| 3 | C3H8 | Propane | C3H6 | Propene |
| 4 | C4H10 | Butane | C4H8 | Butene |
| 5 | C5H12 | Pentane | C5H10 | Pentene |
| 6 | C6H14 | Hexane | C6H12 | Hexene |
| 7 | C7H16 | Heptane | C7H14 | Heptene |
| 8 | C8H18 | Octane | C8H16 | Octene |
| 9 | C9H20 | Nonane | C9H18 | Nonene |
| 10 | C10H22 | Decane | C10H20 | Decene |
The compounds from butene to decene may have branches at equal locations of carbon atoms, so they may also be called but-1-ene, hept-1-ene or dec-1-ene. This means that the unsaturated bond is present between the carbon atoms that start the chain. The phenomenon of the various possibilities of the placement of the unsaturated multiple bond is called positional isomerism. For example, a compound with the molecular formula C7H14 has several possible positional isomers. The double bond in its structure can be located, for example, at the carbon atoms number one and three, as shown in Figure 3.
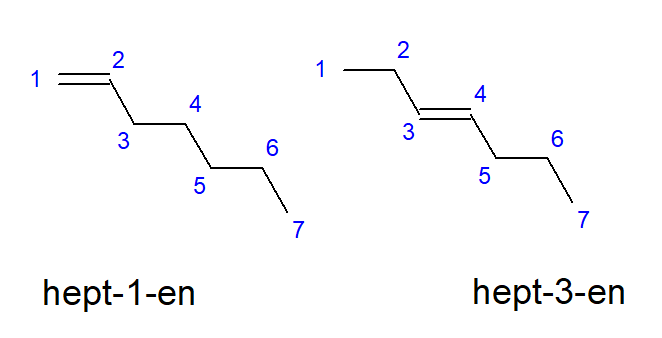
Figure 3. Structural formulas of hept-1-ene (1-heptene) and hept-3-ene (3-heptene)
Structure of alkenes – summary
Each alkene has a double pi bond (π) at which flat structures are created. Carbon atoms have a sp2 hybridisation, characterised by a trigonal form. In saturated locations, the –CH2– methylene groups can create spatially expanded straight and branched chains. Rotation occurs only around the single bonds, and doesn’t occur in the case of the C=C bond.
The basic physical and chemical properties of the group of alkenes
Alkenes have hydrophobic properties and therefore have a non-polar nature. They enter into reactions with water. Because of their affinity, they dissolve well in non-polar solvents such as alkanes. The melting point, boiling point and the density of the alkenes increase as the number of carbon atoms in the molecule increases. Their activity is the largest in short-chain alkenes.
Obtaining of alkenes
There are several ways of obtaining alkenes. Each of these ways is based on the elimination of two substituents at a single bond, as shown on the diagram:
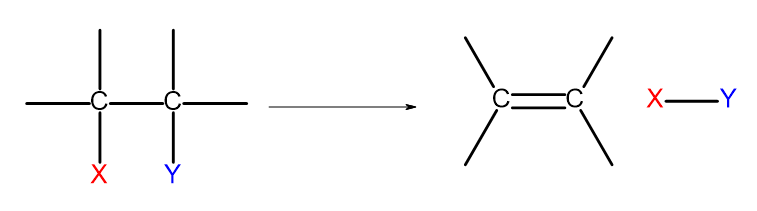
Figure 4. The diagram of the elimination process that results in the formation of an alkene
In general, these are: the reactions of the dehydrogenation of alkanes; the dehydration of an alcohol; the reaction of halogen-alkanes with a strong base; the reaction of dihalogen-alkanes with zinc dust, and the hydrolysis of alkynes.
Obtaining of ethene
1. Dehydrogenation of an alkane
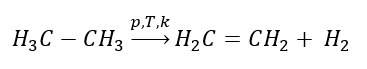
2. Dehydration of an alcohol
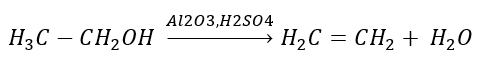
3. The reaction of a halogen-alkane with a strong base
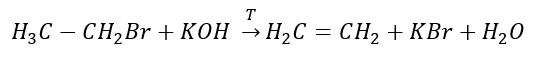
4. The reaction of a dihalogen-alkane with zinc dust
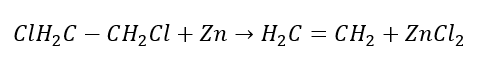
5. Hydrogenation of an alkyne
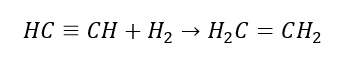
Chemical reactions of alkenes
A particle-specific fragment, which at the same time defines its properties and affiliation, is called a functional group. For alkenes, this group is a double bond (C=C). The pi bond (π) that it contains is very susceptible to breaking. Compared to electrons of the sigma bonds, electrons that occupy the pi orbital are further away from the carbon atoms and therefore are less associated with them. This results in their greater mobility and greater availability for approaching reagents, resulting in a high chemical reactivity of the bond. There are several types of reactions according to which the alkenes are transformed. These are: electrophilic addition, free-radical addition, oxidation and reduction, polymerisation, and allylic substitution.
Electrophilic addition
Because of the nature of electrons of the pi bond, the agents that attack them have an electropositive character. Such molecules are electrophilic reagents which have a high affinity for electrons. In most cases, this factor is a proton of acid origin, an electron gap, or a molecule that is easily polarised as a result of being close to pi electrons. The addition, i.e., the connection reaction, follows the ion mechanism. This is a two-step mechanism whereby an initially approaching electrophilic reagent captures the electrons of the pi bond with the creation of a single bond and the formation of a transient carbocation. This highly reactive molecule rapidly attacks other reagents that have the ability to mutualise electrons. Examples of such reactions are additions of chlorine or bromine to an alkene. Hydrogen may also be added (hydrogenisation) by way of addition, but it is not electrophilic.
Free-radical addition reactions
Many reactions of alkenes show non-compliance with Markovnikov’s rule. Their mechanism focuses on the reaction of electrons of the pi bond to free radicals, during which a carbon-attacking particle bond is produced. As a result of these changes, a new free radical is obtained, which has an unpaired electron at the neighbouring carbon atom.
Allylic substitution
In addition to the reaction involving the double bond, alkenes may also react in a way that is similar to that of alkanes in neighbouring alkyl groups. An example of such a reaction is the substitution of a hydrogen atom with halogen at the atom next to the carbon atom that forms the double bond. This reaction does not affect the unsaturated part of the structure.