Among the organic chemical compounds that are classified as unsaturated hydrocarbons, we can distinguish the group called alkynes. They belong to this group of compounds due to their structure – the molecule contains only atoms of carbon and hydrogen, and one of the bonds between the carbon atoms is unsaturated. For alkynes, this is a C≡C triple bond. Many representatives of the group can be found in everyday products such as petrol, ink, and pesticides, but also in cosmetics, where they have the role of antioxidants.
The structure of alkynes
The general formula for alkynes is CnH2n-2, and each contains a minimum of one triple bond. Structurally, they are isomers of dienes, cycloalkenes and two-ring cycloalkenes. Alkynes, like alkanes or alkenes, have a chain structure that is either straight or branched. Apart from the triple bond, which is a covalent bond, there are only other single but also covalent C-C bonds between carbon atoms and C-H bonds between carbon and hydrogen atoms. The triple bond will only occur if two consecutive carbon atoms are spatially located in a linear sp hybridisation. The length of this bond is approximately 0.120 nm. For example, in ethyne, one carbon hybrid joins with the orbital of a hydrogen atom to produce a sp-s (δsp-s) covalent sigma bond. The second, on the other hand, binds with an analogous sp-sp (δsp-sp) sigma bond resulting in another hybrid joining a hydrogen atom. Such changes result in the formation of the linear framework of the molecule. Due to the resulting sp hybridisation, two p orbitals perpendicular to the bond axis are also present, from which two π bonds between the carbon atoms are ultimately produced. There is no rotation around the triple bond.
Alkynes – nomenclature
There are several basic principles to follow when naming these chemical compounds:
- The presence of an unsaturated triple bond in the structure is indicated by the ‘-yne’ suffix replacing the ‘-ane’ suffix in the name of the relevant alkane homologue. In the case where several locations of such a bond are possible, the ‘-yne’ suffix always precedes the relevant number, indicating the bond locant, such as hept-2-yne or hept-1-yne. The locant may also be placed at the beginning of the name, for example 1-butyne.
- For structures that contain more than one triple bond, a prefix is added to the suffix to indicate the number. These will be -di, -tri, etc., respectively.
- The numbering of carbon atoms must take a direction in which the main chain contains as many carbon atoms as possible as well as the triple bond.
- In the case where the only unsaturated bond is the triple bond, the carbon atoms shall be numbered so that it has the lowest possible number. This rule does not apply if there are other unsaturated bonds (double), in which case their locants must be as low as possible.
- In the name of a compound with one triple bond next to the first locant, the number can be omitted, in other words, the name prop-1-yne may be used as well as the name propyne
The physico-chemical properties of alkynes
The nature of alkynes is non-polar, they are hydrophobic, and they react with water. Preferably, they dissolve in similar non-polar solvents such as alkanes. As the carbon chain elongates, their melting point, boiling point and density increase. However, their activity decreases at the same time. The most reactive are those with the least carbon atoms in the chain. Compared to alkanes and alkenes, they are slightly more reactive due to the triple bond being less than a single or double bond. Their molecular structure also results in the high flammability of these compounds. One of the characteristic reactions of alkynes is the reaction of combustion. They are capable of adding an electrophilic reagent at the place of the unsaturated bond. They undergo polymerisation reactions.
Alkyne homologous series
Like alkanes and alkenes, alkynes also have their own homologous series, i.e., their hierarchy of basic compounds, which contain exactly one triple bond classified according to the increasing number of carbon atoms in the structure. The compound that starts the alkyne homologous series is ethyne, also called acetylene, which has two carbon atoms and two hydrogen atoms in its structure
| Number of carbon atoms in the chain | Alkane formula | Alkane name | Alkyne formula | Alkyne name |
| 2 | C2H6 | Ethane | C2H2 | Ethyne |
| 3 | C3H8 | Propane | C3H4 | Propyne |
| 4 | C4H10 | Butane | C4H6 | Butyne |
| 5 | C5H12 | Pentane | C5H8 | Pentyne |
| 6 | C6H14 | Hexane | C6H10 | Hexyne |
| 7 | C7H16 | Heptane | C7H12 | Heptyne |
| 8 | C8H18 | Octane | C8H14 | Octyne |
| 9 | C9H20 | Nonane | C9H16 | Nonyne |
| 10 | C10H22 | Decane | C10H18 | Decyne |
Obtaining of alkynes as seen with ethyne
- The reaction of carbide with water:
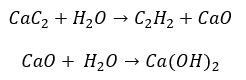
- Pyrolysis:
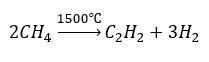
- High-temperature synthesis:
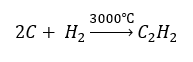
- Partial oxidation of natural gas:
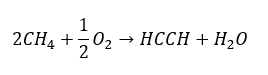
Higher alkynes are obtained on an industrial scale by using as reagents ethyne and, for example, formaldehyde in the condensation reaction. Another method is the two-fold elimination of hydrogen halide from relevant alkyl halides that contain two halogen atoms and are attached to one carbon atom or to adjacent carbon atoms. In the latter case, there are two possible products: an alkyne and a diene. Below is an example of a reaction of propyne formation:
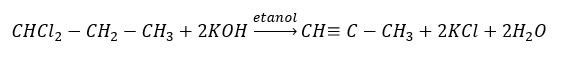
Reactions characteristic for alkynes:
- Total combustion with the generation of carbon dioxide:
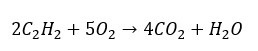
- Partial combustion with the generation of carbon monoxide or soot:
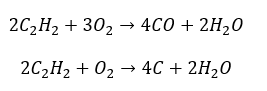
- Hydrogenation reaction in the presence of metallic catalysts (Pd, Pt, Fe, Ni), resulting in the formation of alkenes. It may occur in stages, with successive individual hydrogen molecules being added, or all at once, with alkane formation:
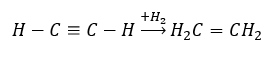
- The Kucherov reaction, typical for alkynes, involves the addition of a water particle in the triple-bond area, which takes place in two stages. Initially, an unstable enol is formed, which is a combination of an alkene and alcohol. It then undergoes the keto-enol tautomerism, i.e., a regrouping with the formation of aldehydes or ketones. The conversion is carried out by way of an electrophilic mechanism:
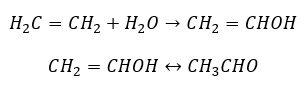
- The electrophilic addition reaction with HCl or HBr, occurring in accordance with Markovnikov’s rule. It may occur in whole or in multiple stages.
- The electrophilic addition of bromine, with the production of di- or tetra- bromo-derivatives. Because of the reactions with bromine, alkynes discolour bromine water. They also react with chlorine particles.
- Free-radical fluoride addition reaction, which is carried out under the influence of light energy.
- Substitution reactions, where the hydrogen atom at the triple-bond carbon atom is displaced by a metal atom.
- Trimerisation, i.e., polymerisation with three molecules, using acetylene, which allows the synthesis of benzene.