The term “element” refers to atoms whose nuclei contain a particular number of protons. Apart from them, an atomic nucleus also consists of other molecules: neutrons. Their number within a single element may vary. In such a case, we talk about isotopes, i.e. variants of a given element that have different numbers of neutrons in their nucleus. Thus, their mass numbers are also different. However, the atomic number remains constant, as the number of electrons on electron shells remains unchanged.

For this reason, the isotopes of the same element have the same position in the periodic table, and each of them has the same size. Also, their chemical and physical properties are similar. However, there are exceptions where especially the physical properties are more diversified, which usually occurs when we deal with two isotopes with significant differences in mass. This is due to the fact that it is the mass that determines a whole range of properties such as density or particle diffusion speed. In contrast, parameters such as electrical conductivity or colour do not depend on mass. The diversity of chemical properties normally comes from unequal speeds of reaction of various isotopes.
Nuclides vs isotopes
There is a concept associated with isotopes, which is more generic. A nuclide is an entire set of atoms whose nuclear structure is specifically determined by the number of protons and neutrons. This means that two nuclides which differ in the number of neutrons can be isotopes. In practice, all isotopes are nuclides but not every nuclide is necessarily an isotope. The main idea behind these two concepts covers chemical properties in the case of isotopes and nuclear properties in the case of nuclides. For example:
- Nuclides which are isotopes:
- Nuclides which are not isotopes:
Isotopes in nature
Chemical elements existing in the natural environment are a mixture of isotopes with a constant percentage composition. Depending on the nucleus, an element can contain one, several or more isotopes. Some of them are stable, but some quickly transform. Such a decomposition may produce isotopes of the same or a different element. Such transitions usually go along with the emission of radiation. To use particular values, on Earth there are elements whose atomic numbers are equal to or lower than 92. Those with numbers up to 83 are considered stable. There are as many as 81 naturally existing elements that have stable isotopes. These include silicon, with isotopes 28Si, 29Si and 30Si, iron, with isotopes 54Fe, 56Fe, 57Fe and 58Fe, or aluminium, which has only one stable isotope: 27Al.
Hydrogen isotopes
In nature, we can find three hydrogen isotopes that form an element mixture. In practice, the isotopes 1H (hydrogen-1) and 2H (hydrogen-2) represent a majority of, respectively, 99.985% and 0.015% of the total occurrence of hydrogen. The isotope 3H (hydrogen-3) is unstable and represents only some trace amounts. Hydrogen-1 is the best known protium, hydrogen-2 (also called deuterium) is made of one proton and one neutron, while the nucleus of the last isotope (hydrogen-3, i.e. tritium) contains one proton and two neutrons. For this reason, the difference in mass between these isotopes is significant: the atom of deuterium is twice as heavy as that of protium, while tritium is even three times heavier than protium. As we know, hydrogen in any isotopic form exists as a diatomic molecule. It turns out that it may also form molecules containing different isotopes, i.e. HD (protium-deuterium), HT (protium-tritium) and DT (deuterium-tritium). Deuterium is a non-radioactive, high-stability isotope. It is sometimes referred to as heavy hydrogen. If we replace hydrogen-1 with deuterium in a molecule of water (D2O), the molecule will change its properties: the melting point will rise by around 1.5oC and the freezing point will drop by as many as 3.81oC. Its density will also increase relative to H2O by around 0.1%. In contrast, tritium is a highly unstable isotope that is also radioactive. Its nucleus is a place of spontaneous radioactive decompositions that produce helium atoms. If we compare the physico-chemical properties of isotopes, we will find essential differences, which are shown in Table 1.
Table 1. Comparison of basic physico-chemical parameters of hydrogen isotopes.
| Isotope | Symbol | Density [g/l] | Melting point [K] | Boiling point [K] |
| Protium | H | 0.08233 | 13.83 | 20.27 |
| Deuterium | D | 0.1645 | 18.73 | 23.67 |
| Tritium | T | 0.2464 | 20.62 | 25.04 |
Carbon isotopes
Carbon has three known isotopes: 12C, 13C and 14C, each of them having the same chemical properties. The most common one is carbon-12, which represents as much as 98.89% of all atoms. The 13C isotope existing on Earth represents around 1.11%, while the 14C atom occurs in one per around 1012. The latter one is predisposed to a spontaneous decomposition, which causes the emission of beta radiation. Then the isotope transforms into a nitrogen atom. A stable isotope is carbon-13. Owing to its non-zero spin, it can be applied in 13 NMR nuclear magnetic resonance.
Application of isotopes
In smoke detectors, we use unstable isotopes, 241Am or 238Pu, which are characterised by a slow decomposition combined with the emission of radiation. As smoke appears, the radiation is blocked and no longer reaches the detector, thus triggering the alarm. The decomposition radiation of some elements is capable of destroying microorganisms, and therefore isotopes, including 60Co, may be used for preserving food. In medical diagnostics, certain nuclei are used to detect changes in the tested organs, for example in kidneys or in the heart. Such an isotope combines with a biologically inactive substance with a known path in the body and introduces it to the blood circulation system. This makes it possible to track the path of the radiation and observe as it accumulates in tissues and organs. In tumour treatment, we also apply radiotherapy based on radiation emission by unstable isotopes such as 226Ra and 60Co. The goal of such a therapy is to destroy tumour cells. One of the carbon isotopes, 14C, is applied for assaying the age of materials of organic origin. This unstable nucleus slowly decomposes, and if the organism dies (which is when the assimilation of carbon stops), its concentration of carbon-14 is reduced in proportion to the passing years. Some isotopes, for example 239Pu and 235U, are used in nuclear power plants. During the decomposition of those unstable nuclei, energy is produced which in turn may be converted into electricity.
Atomic mass and isotopes
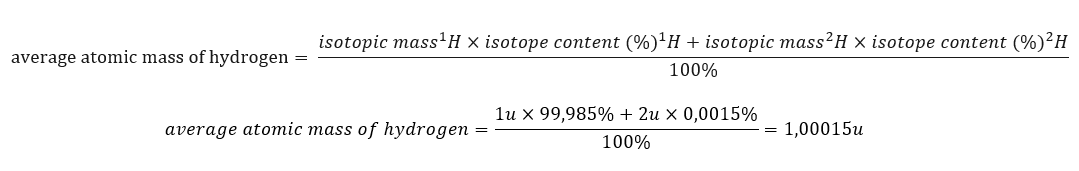
The atomic mass of elements presented in the periodic table is actually the averaged mass. When we calculate it, we take into account the number (n) and percentage composition (xn) of each isotope of the element in question, according to the following formula:
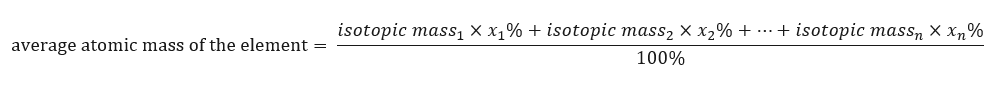
For example, when calculating the average atomic mass of hydrogen, we will obtain the following equation: