Peptide bond is one of the most important bonds existing in nature. It interlinks individual molecules of amino acids to form peptide and protein structures. In addition, it shows unique properties owing to the existence of, for example, mesomeric forms, or the possible rotation of functional groups around the carbon-nitrogen bond.
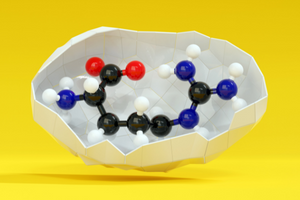
Structure of peptide bond
Peptide bonds (-CO-NH-) are some of the most important bonds existing in nature. They are composed of the atoms of carbon, oxygen, nitrogen and hydrogen. These bonds are formed by the reaction of condensation between the carboxyl group (-COOH) and the amino group (-NH2). The reaction most often occurs between two different or identical amino acids. Its by-product is the molecule of water. A peptide bond is decomposed as a result of hydrolysis. It is then split, and the different amino acids are reconstructed. Hydrolysis takes place at an increased temperature, in water environment as well as in the presence of concentrated inorganic acids or concentrated bases.
Peptide bonds are flat and cannot rotate between the carbonyl carbon and the atom of nitrogen. However, such bonds have mesomeric character, which means that there are two mesomeric forms being the result of the ‘movement’ of a double bond in the peptic bond. In consequence, the carbon-nitrogen bond partly shows the features of a double bond, which improves its chemical stability. Peptide bonds exist in two isomeric forms: cis and trans (the peptide bond in proteins and peptides is most often trans).
Polypeptides
Based on how many amino acid molecules interlink through peptide bonds, we can distinguish:
- dipeptides, which are made of two amino acid molecules,
- tripeptides, which are a combination of three amino acid molecules,
- oligopeptides, which contain fewer than 10 amino acid residues,
- polypeptides, which contain 10 to 100 amino acids, and
- proteins, which are high-molecular structures containing more than 100 amino acid molecules.
The combination of more than two amino acid molecules leads to the formation of polypeptides. Two amino acid molecules combined form a peptide bond. Once combined, amino acids have free functional groups that can create further bonds with other amino acid molecules. This is how polypeptides are formed.
Detecting peptide bonds in a biuret reaction
The biuret reaction is a characteristic reaction that signals the presence of peptide bonds. However, it can be used not only to detect peptide bonds but also to determined them by quantity. This is particularly useful for chemical compounds in which peptide bonds are located close to one another. Such compounds include peptides or proteins. In a biuret reaction, it is possible to detect at least two peptide bonds. This makes that method inappropriate for detecting amino acids (which have no peptide bonds) or dipeptides (which have only one peptide bond).
To detect a peptide bond, we must first create a basic reaction environment by adding a solution of a strong base (it can be sodium hydroxide or potassium hydroxide). This makes it possible to form a coloured complex of copper(III) ions. Then the tested solution is complemented with copper (III) sulphate with an intensive blue colour as well as potassium sodium tartrate (which maintains an adequate solubility of the whole complex). Peptide bonds with copper(III) ions form a coloured complex, which can be analysed spectrophotometrically with the wave length of 546 nm (absorption maximum). If the colour changes from blue into violet, it indicates that the tested material contains the peptide bond. The colour intensity depends on the quantity of peptide bonds.
Amino acids, peptides and proteins
Amino acids, peptides and proteins have one common denominator, which is the peptide bond. All the structures mentioned above play a very important role in the world of nature and in the proper functioning of our body.
Amino acids are compounds with a relatively complex molecular structure. From the chemical point of view, they are organic compounds that have at least one amino group and carboxyl group. Their lateral chains can be linear, circular, or branched. Amino acid molecules bind with one another to form dimers and polymers with various chain lengths and various compositions.
Peptides are structures that contain around 50 (and a maximum of 100) amino acids, for this reason they are often called short chain proteins. They take part in all physiological processes, acting as regulators and transmitters. They are produced by condensation between a carboxyl group and an amino group, that is, with the formation of a peptide bond. The by-product of that reaction is a molecule of water.
Proteins are high-molecular molecules with a complex design. They contain a range of amino acids which are interlinked via peptide bonds in various sequences. Amino acids combine to form proteins in such a way that the carboxyl group of one amino acid bonds with the amino group of another amino acid or with the amino group of another molecule of the same amino acid; this creates the so-called dipeptide, which has a free amino group and carboxyl group. This makes it possible to add further amino acid molecules and form polypeptides.