It is one of two principal divisions of analytical chemistry, which deals with detecting chemical elements, functional groups or ions existing in the tested structure. In terms of the methods used, we can divide chemical analysis into classical analysis and instrumental analysis. Classical analysis relies on methods based on chemical reactions which may be carried out in “dry” or “wet” mode. In contrast, instrumental analysis relies on instruments, that is analytical measuring instruments with the main element being the detector.

Classical analysis: dry reactions
The term “dry reactions” refers to the changes that occur in chemical compounds while they are subjected to high temperatures. We distinguish three principal types of such reactions:
- melting the tested sample with solid fluxes,
- obtaining borax or microcosmic salt beads,
- colouring the gas burner flame.
The most popular is the third method, which is referred to as the flame test and allows for detecting many elements. This is an example of a technique that uses radiation emission. It consists in examining the characteristic radiation that is emitted by the atoms of particular elements once they are excited by high temperature. This is since it has been demonstrated that such conditions cause evaporation of the compounds of certain metals, and the vapours produced are excited and colour the burner flame in a characteristic way. The colour is an effect of the excitation of particular atoms which, while being restored to their original state, emit a light-quantum that refers to a specified wave length. For instance, the flame colours characteristic of particular elements are as follows:
- sodium: intensive yellow,
- potassium: violet,
- calcium: brick-red,
- barium: green,
- bismuth: light blue.
Classical analysis: wet reactions
These are all types of reactions that occur between the tested samples and solutions of various chemical reagents. To carry out such reactions, the tested substance must be converted into a solution. The applied reactions are selected based on many criteria, so that they:
- show high sensitivity, i.e. occur already at low concentrations of the substance being detected;
- occur in a short time and show easily observable changes such as a change in the colour of the solution, in precipitation, or in gas emission;
- are selective, i.e. occur only within a known group of ions.

Qualitative analysis: inorganic chemistry
In inorganic chemistry, qualitative analysis revolves around two topics: the detection of cations and anions. Inorganic chemistry uses characteristic reactions that involve appropriate group reagents. They are called “group reagents” because the cations have been divided into five categories. Such a reagent forms sediments with only one of them, which makes it possible to narrow down the types of cations present in the sample according to the following pattern:
- Group 1: Ag+, Hg22+, Pb2+ – group reagent 3M HCl;
- Group 2: Hg2+, Cu2+, Cd2+, Bi3+, As3+, As5+, Sb3+, Sb5+, Sn2+, Sn4+ – group reagent H2S in an 1M HCl environment;
- Group 3: Ni2+, Co2+, Fe2+, Fe3+, Mn2+, Zn2+, Al3+, Cr3+ – group reagent (NH4)2S in an ammonium buffer environment;
- Group 4: Ca2+, Sr2+, Ba2+ – group reagent (NH4)2CO3 in an ammonium buffer environment;
- Group 5: Mg2+, Na+, K+, NH4+ – no group reagent.
Once we exclude certain groups of cations, we can continue the identification with the use of further reagents, this time ones that are characteristic of particular ions. Such a reaction allows for an unambiguous identification. For example, the detection of Ag+ ions in a sample occurs in two stages:
- The reaction with a group reagent: production of a white sediment
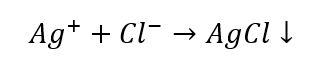
- The characteristic reaction: dissolving the AgCl sediment in an aqueous ammonia solution, obtaining a colourless complex compound.
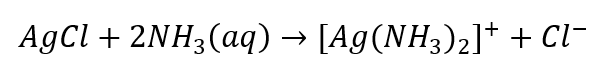
We can identify anions, which are divided into three groups, in a similar way:
- Group 1: BO2–, CO32-, C2O42-, SiO32-, PO43-, AsO33-, AsO43-, SO32-, S2O32-, SO42-, F–, CrO42-, Cr2O72- – group reagent BaCl2, the formation of salts sparingly soluble in water;
- Group 2: C4H4O62-, S2-, Cl–, ClO–, Br–, I–, CN–, SCN– – group reagent AgNO3, the formation of salts sparingly soluble in water and in diluted nitric acid;
- Group 3: CH3COO–, NO2–, NO32-, ClO3–, ClO4–, MnO4– – the group reagent contains silver or barium cations; the formation of water-soluble salts.
The identification of cations is somewhat more problematic compared to anions, as the sequence of procedures depends on the outcomes of group reactions, plus there are ions that disturb the identification process. For instance, to detect a CO32- ion we have to conduct the following reactions:
- The Ba2+ ions produce a white sediment that is soluble in acids
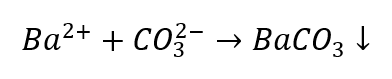
- Diluted acids cause decomposition with CO2 precipitation
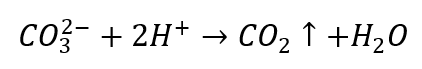
- Carbon dioxide causes the solution to bubble. It can be covered with limewater, as the white sediment will precipitate.
* Disturbing ions: SO32- and S2O32- also form white sediments with the calcium cation. They must be oxidated to eliminate the disturbance.

Qualitative analysis: organic chemistry
The qualitative analysis of organic compounds includes multiple stages, and the key point is to solve five fundamental issues:
- Assaying of physical parameters such as the melting or boiling point. Unfortunately, there are many chemical compounds with the same temperature points, and the measurement itself may be affected by error. However, if we have an appropriate reference standard, this method may allow us to quickly identify the compound. In addition, by measuring the temperatures we can determine the compound’s purity, as the temperature ranges are narrow. The constancy of Ttop after at least one crystallisation may also suggest high purity of the compound. For liquids, it may indicate a narrow distillation range.
- Assaying of elemental composition can exclude or confirm the presence of particular types of organic compounds. To give an example, by excluding the presence of nitrogen atoms in the structure, we also exclude the presence of amino or nitric groups. To this end, we carry out characteristic experiments such as the Lassaigne’s test for nitrogen, Beilstein’s test for halogens, or the sulphur test using sodium nitroprusside.
- Testing the solubility of the compound allows us to classify it into the group of compounds with specified chemical properties. According to the “like dissolves like” principle, compounds have been divided into 7 categories.
- Identification of functional groups requires appropriate analytical reactions that make it possible to exclude or identify functional groups.
- Spectral analysis is the most reliable point that enables us to clearly identify a chemical compound. It covers all instrumental techniques, such as:
- Mass spectrometry (MS), which consists in ionising the molecules and detecting the number of ions by determining their mass-to-charge ratio. This allows us to draw conclusions about the mass of the analyzed compound;
- Nuclear magnetic resonance (NMR) spectroscopy, which delivers specific information about the structure. It provides an image of magnetic nuclei (13C, 1H), which enables a thorough interpretation of their quality;
- Infrared (IR) spectroscopy uses a narrow range of electromagnetic radiation to show the types of vibrations existing in the tested molecule.