Spectroscopic methods are a group of methods in which signal is produced by the interaction of electromagnetic or corpuscular radiation with the examined sample. This radiation can arise as a result of periodic changes in the electromagnetic field associated with energy transfer. These methods are used to determine the concentration or content of atoms in a given absorbing or emitting system. What types of spectrophotometry are there and what do these methods involve? This is what you will find out from our article.

Radiation and energy transitions
The most important property of photons, or radiation quanta, is energy. The formula for the energy value (E) is the product of Planck’s constant (h) of 6.626·10-34 [J·s] and radiation frequency (v) expressed in Hertz [Hz].
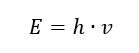
Spectrophotometry makes use of energy transitions in molecules, caused by the absorption or emission of electromagnetic radiation in different ranges:
- ultraviolet (UV) 200-380 nm,
- visible radiation (Vis) 380-780 nm,
- near infrared (IR) 0.78-30000 μm.
Basic concepts and laws of spectrophotometry
Isosbestic point
This is the point corresponding to a specific wavelength resulting from the intersection of curves plotted in the absorbance-wavelength system, where both forms of the compound in solution (dissociated and undissociated) have equal absorption. A change in pH does not change the position, a compound may have many such points.
Absorption spectrum of a chemical compound
The spectrum is a measure of the amount of light absorbed by the compound from the light wavelength (λ). If electromagnetic radiation (light) of intensity I0 falls on a sample, some of this radiation will be absorbed and some will pass through the sample. By recording the intensities of incident (I0) and transmitted (It) radiation, we can determine the amount of light that is absorbed by the solution (absorbance, A), or that has passed through it (transmittance, T).
Absorbance
The quantity that describes the phenomenon of absorption is absorbance. This is a dimensionless measure of the intensity of electromagnetic radiation relative to the number of free atoms. This figure was introduced to facilitate calculations related to absorption volumes. Absorption is the interaction between electromagnetic radiation and matter – the absorption of some of the energy by matter. Absorbance is additive and is sometimes referred to as optical density. Symbolically, it is recorded as Abs or A. Mathematically, it is the decimal logarithm of the ratio of the intensity of the beam of radiation incident on the test sample (I0) to the intensity of the beam of radiation transmitted through the test sample (It). It can take values from 0 to infinity. The mathematical expression describing this relationship is called Beer-Lambert law. Absorbance at a given wavelength depends on the absorption coefficient (ε), the thickness of the absorbing layer (usually 1 cm) and the concentration of the test solution.
Transmittance
Transmittance is the ratio of the irradiance passing through the sample (It) to the irradiance incident on the sample (I0), which is equal to the irradiance passing through the reference. It is most often expressed as a percentage and can range from 0% to 100%. It is expressed by the formula:
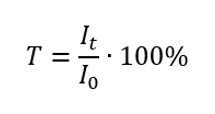
The relationship between absorbance and transmittance is expressed as follows:
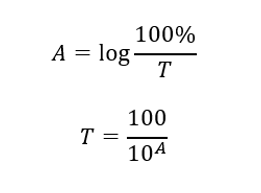
Lambert Law
It says that the absorbance is proportional to the thickness of the absorbing layer of a homogeneous sample. It can be recorded as the quotient of the absorption coefficient (k) and the thickness of the measured sample layer (l):
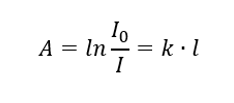

Beer-Lambert law
It concerns the absorption of radiation by solutions and can be formulated as follows: if the absorption coefficient of the solvent is zero, then the absorbance of a monochromatic radiation beam passing through a homogeneous solution is directly proportional to the concentration (c) of the solution and to the thickness of the absorbing layer (l). Beer-Lambert law is the definition of absorbance and can be written as follows:
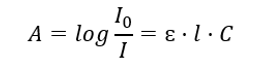
The molar absorption coefficient (ɛ) is a characteristic value of a substance in a particular solvent and at a particular wavelength. It is proportional to the probability of transitions between the energy levels of the molecule and directly depends on the energy of the radiation photons. In numerical terms, it represents the absorbance exhibited by a solution with a concentration of 1 mol/dm3, with an absorbing layer thickness of 1 cm. Knowing the coefficient makes it possible to determine the concentration of the test solution after measuring the magnitude of the absorbance. Optionally, the expected absorbance of the solution can also be calculated from the coefficient and concentration. The formula is mainly used to determine the concentration of a substance with a known molar absorption coefficient and empirically measured absorbance. This can be done using a formula or by drawing a calibration curve for the substance at several points in the range of expected concentration. Beer-Lambert law can also be presented as concentration dependence of absorption: A = f(C).
Law of additivity
Beer-Lambert law refers to the case where there is one absorbing substance in solution. However, if there are more substances in a multicomponent solution that absorb radiation at a selected wavelength, the absorbance of this solution (A) is equal to the sum of the absorbances of its individual components (A1, A2,…), i.e. A=A1+A2+⋯+An. It is worth noting and remembering that the absorbance of each component is the product of its concentration and the corresponding molar absorption coefficient. This is the 3rd law of absorption, used in spectrophotometric analysis of multicomponent systems.
Deviations from the laws of absorption
A prerequisite for meeting the laws of absorption is that the radiation acting on the system is monochromatic and that its intensity is not too high. The reasons for deviating from Beer-Lambert law can be:
- imperfection of measuring instruments, resulting in the condition not being met,
- chemical reactions occurring during the measurement, for example polymerisation, hydrolysis, condensation,
- turbidity of the solution.
Application of electron absorption spectra
- It allows for the type of electron transitions to be determined by examining spectra in solvents of different polarity.
- The differences in the position and intensity of the absorption bands of the different molecular forms are used to study the equilibria established between them in the solution (structural studies, tautomeric equilibria).
- Used for compound determination based on Beer-Lamber law.
- Used as an additional identification method based on the λmax position of test substances.
- Used in the determination of the purity of test compounds – shifting the absorption maximum position, comparing the ratio of absorbance values at two different points in the spectrum.

UV-Vis electron spectroscopy
In terms of the phenomenon of absorption, UV-Vis spectrophotometry is a particularly interesting technique, allowing qualitative and quantitative analysis of many substances. The radiation absorption phenomenon is used when: absorption depends linearly on concentration or when absorption is additive, i.e. for a multicomponent solution it is the sum of the absorptions of its components. The conditions are met when there are no intermolecular interactions in the system.
By analysing the UV-Vis absorption spectra resulting from the passage of electromagnetic radiation through the solution, transitions of valence electrons from the ground to the excited state are observed. This phenomenon results in the absorption of part of the radiation at certain wavelengths. Absorption is the consequence of the transition of these electrons to higher energy levels, but in order for this to happen, the quanta of electromagnetic radiation must have enough energy to balance the energy difference between the energy levels. In molecules, such energies are a few electronvolts, which corresponds precisely to the UV-Vis frequency.
Chromophores
Compounds that show absorption in the UV-Vis range have chromophores in their structure, i.e. groups of atoms whose electrons are characterised by low excitation energies. In other words, a chromophore is the part of a molecule (group of atoms) responsible for the selective absorption of radiation in the visible range (180-800 nm) and thus for the occurrence of colour. These include: aromatic rings (aromatic electron sextet), multiple bonds (part of them – π-type bonds), both between carbon atoms and others, such as the carbonyl group C=O.
Auxochromes
Another group of atoms are auxochromes, i.e. substituents that do not show absorption in the UV-Vis range, but their presence causes changes in the spectra of the absorbing elements. They enhance the action of the chromophores, which we can often observe in the form of a much more intense colouring of the test substance than in case of their absence in the system. In addition, we divide the auxochromes into bathochromes, e.g. –NH2, -OH, which shift the absorption maximum towards longer wavelengths, and hypsochromes, such as –CH3, -CO, which shift the maximum towards shorter wavelengths.
Atomic Absorption Spectroscopy (AAS)
This is another technique using the phenomenon of absorption. It is used to determine chemical elements in the form of samples in any state (liquid, solid, gas) and the measurement itself is based on observing the absorption of radiation of a specific wavelength by free metal atoms. The basis of the technique is that an atom can only absorb electromagnetic radiation at a wavelength at which it can also emit it, and this is characteristic only of the given element. With higher energy supplied to the atom, the electrons are excited to higher levels and a greater number of lines is observed in the spectrum. If the energy supplied corresponds to the ionisation potential of the atom, ions such as Na+ are formed. The basis for quantitative analysis by atomic absorption spectrometry is the proportionality of absorbance to the number of absorbing atoms and Beer-Lambert law.