The pH scale is used to determine the degree of acidity or alkalinity of a solution on a quantitative scale that contains the absolute numbers from 0 to 14. It is based on the molar activity of hydronium ions [H3O+] in the tested liquid. The scale was developed in 1909 by the Danish biochemist S. Sorensen, and the letters ‘p’ and ‘H’ stand for, respectively, the Latin ‘potentio’ (power) and the atom of hydrogen, which is denoted in the periodic table as ‘H’ (hydrogenium). The pH scale reflects both the one and the other: to determine the pH level, we use the power exponent with a changed sign to refer to the protons containing hydrogen. The pH level of any solution produced is one of its most important chemical features and a factor that determines the course and speed of many chemical reactions as well as the type of the substances produced.

Differentiation on the scale
The pH scale includes fifteen degrees, where the middle value (7) indicates a neutral pH. Solutions positioned close to 0 are called strong acids, while those on the opposite side, close to 14, are referred to as strong bases. An acidic pH means that the solution contains too many hydrogen ions [H3O+], and a basic pH means that there are too many hydroxide ions [OH–]. The pH scale is most often referred to in the context of solutions, but the absolute chemical potential of a proton makes it applicable in all states of aggregation. This allows us to directly compare the alkalinity level of virtually any substance.
Self-dissociation of water
To fully understand the sense of the pH scale, we need the spontaneous reaction of water self-dissociation, which proceeds according to the following equation:

It is a reversible process whose equilibrium constant is shifted to the left side of the equation, which is to say, towards undissociated water.
How to calculate the pH level?
Despite the erroneously defined initial assumption that the determination concerns the concentration of hydrogen ions [H+], the most popular formula is still the following:

Today we already know that solutions do not contain aqueous hydrogen ions, i.e., protons present in water, due to the immediate process of solvation. The phenomenon results in the lack of free protons and the presence of hydronium ions [H3O+], which is caused by the spontaneous, irreversible reaction:
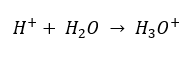
Therefore, the correct notation of the formula is as follows:
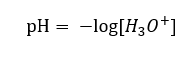
Environment denoted on the pH scale
It is assumed that the concentration of hydronium ions in pure water at room temperature (25 oC) is 10-7 mol/dm3, so its pH level can be calculated as follows:
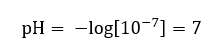
This is in line with the fact that water, being neutral, is positioned in the middle of the scale, which indicates chemical inertness of the substance. It also proves that water contains an equal number of hydroxide anions and hydronium cations. In acids and bases, however, that equilibrium is disturbed. A negative exponent suggests a relation where the lower the pH level, the higher the power of the tested solution. The term ‘power’ in the context of the pH scale refers to the fact that value 0 is taken by strong, even irritating acids, while value 14 refers to the strongest alkaline substances.
The addition of acid into water
A change of pH from a neutral level can be caused by adding substances of different powers. For instance, if we add one of the strongest acids (hydrochloric acid, HCl) to water, it will cause the acid to dissociate according to the following reaction:

Unlike the self-dissociation of water, the equilibrium of acid dissociation is significantly shifted to the right. Therefore, if that acid is dissolved in environmentally inert water, it will increase the quantity of hydronium ions present in the water and, in consequence, increase their molar activity. To give an example, according to the dissociation reaction, hydrochloric acid concentrated at 1 mol/dm3 introduces to the solution hydronium ions concentrated at 1mol/dm3. The pH level for such a hydrochloric acid solution can be calculated based on the following formula:
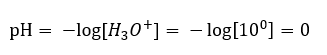
The addition of a base into water
In the opposite event, where we add a strong base into water, the concentration of hydronium ions will drop. For example, sodium hydroxide concentrated at 1mol/dm3 will, analogously, have the same concentration but of other ions (hydroxide ions), according to the reaction it undergoes under the influence of water:

In such a case, it is possible to indirectly calculate the pH using the following supplementary pOH formula:
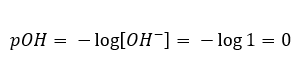
If we know that:
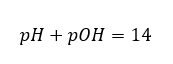
we can easily calculate that the pH of the solution of a strong base (NaOH) concentrated at 1 mol/dm3 has a pH of 14.

The pH level off the scale?
Since the pH scale was originally developed for the purposes of diluted solutions, it is possible that strong acids or bases exceed it, going below 0 or above 14. This is because the scale is useless for high concentrations where the pH is no longer a logarithmic function of hydronium ions [H3O+]. Then we use different values, which arise from constant equilibriums of the dissociation of acids and bases.
Methods of determining the solution’s pH value
To empirically determine the pH of an environment, we use the so-called acid-base indicators. In practice, they are substances that change their colour when affected by different pH conditions. There are three types of such indicators:
- Indicators which change their colour in a basic environment, for example:
- colourless phenolphthalein becomes raspberry-coloured,
- colourless thymolphtalein turns blue.
- Indicators which change their colour in an acidic environment, for example:
- methyl orange changes its colour from orange to red,
- bromothymol blue changes its colour from blue to yellow.
- Universal indicators, for example:
- bromothymol blue turns yellow in acids and blue in bases,
- lacmus turns red in acids or blue in bases.
It is also common to soak litmus papers in a mixture of different indicator substances, which increases the range of the pH levels. Additionally, many laboratories use ready-made, universal litmus papers that change colours in the range from red to green, appropriately suggesting the tested pH based on an accompanying scale. Such determination allows us to estimate only the pH value with an accuracy of 0.5 unit on the pH scale.
A more precise method of measuring the pH value is the acid-base titration, which includes the subtypes of alkalimetry (titration with a standardised base solution) and acidimetry (titration with a standardised acid solution). It uses a titrant with a known concentration, which, when reacting with the tested substance, continuously varies the concentration of hydronium ions present in the solution. Such determination may be carried out in two ways: visually, when determining the end point, along with the colour change or with the use of instrumental methods, for example potentiometry or conductometry.
As the name suggests, the use of a pH meter also enables us to define the substance’s pH value. The instrument, operating based on potentiometry, has a cell in two identical electrodes. One of them, called the indicator electrode, should be put into the tested solution. The other one (comparative electrode) is placed into the standard solution with a known pH value. A commonly used substance is the silver chloride solution. Both electrodes are connected with an extremely sensitive voltmeter, which continuously converts the EMF (electromotive force) into a particular result in the pH scale. According to the Nernst theory, the cell’s electromotive force (EMF), consisting of two identical electrodes immersed in solutions with different pH values, is directly proportional to the logarithm of the quotient of both concentrations.
Home methods of measuring the pH value
It turns out that there are many natural pH indicators, and the approximate pH value can be measured in the home environment. For instance, an infusion made of black tea leaves gets brighter when mixed with an acid, e.g., lemon juice. Conversely, it gets darker under the influence of bases such as a solution of baking soda. Red cabbage juice changes its colour from violet to red when subjected to an acid, or to blue when mixed with a base.